Valence electrons are those in the outermost electron shell of an atom. These special electrons determine the atoms reactivity because they are free to form bonds with the valence electrons of other atoms. An atom's life goal is to achieve 8 electrons in its outer shell through bonding with other atoms. When the atom has 8 electrons in it's outer shell it is stable.
Valence is important when determining how to draw Lewis Structures.
Let's try to start from the beginning. I haven't been in a chemistry class for a while, so this is going to be basic, but hopefully helpful.
Okay, so you may know that an atom is comprised of protons, neutrons and electrons. The protons and neutrons form the atom's nucleus, while the electrons float around it.
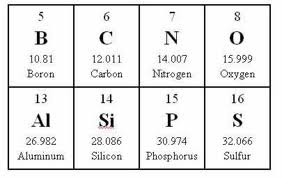
If you look at an element on the periodic table, you will see that each one has an atomic number.
Carbon's atomic number is 6. Nitrogen's atomic number is 7. The atomic number also represents the number of protons and electrons of the element. For example, Carbon is made up of 6 protons and 6 electrons. Likewise, Nitrogen has 7 protons and 7 electrons.
We are trying to determine valence electrons so let's do it.
Things get kind of tricky, the higher you go up the periodic table so we are going to stick with the lower numbers just so you understand the concept of how the valence is determined.
Look at my little ghetto drawing above. Microsoft Paint, what!
Anyway, as you can see the protons/neutrons form the nucleus in the middle and there are electrons hanging about on the surrounding rings.
The first electron ring can hold 2 electrons. The second electron ring can hold 8. And the third ring will hold 8 (sometimes more, but we're going to keep it simple).
Carbon.
Carbon has 6 electrons (look at the atomic number). This means that we have 6 electrons to place in the surrounding electron shells.
Therefore, there will be 2 electrons in the first shell and 4 electrons in the second shell. Since there are 4 electrons in the outer shell Carbon's valence is 4.
How about another?
Oxygen has 8 electrons. This means that 2 electrons would go in the first electron shell and 6 electrons would go in the outer shell. This is why Oxygen has a valence of 6.
Let's do one with a higher number. Phosphorus.
Phosphorus has 15 electrons. So it has 2 electrons in its first shell, 8 electrons in the second shell and 7 electrons in its outer valence shell.
Get it? Read through and look at the diagram above and you'll have it!

No comments:
Post a Comment