Here are the answers to the equations in my last post: Balancing Chemical Equations Step by Step. When you balance equations, the most important thing is to keep an accurate count of all of your atoms as you go through the process. This becomes even more important when you have to balance molecules that have parentheses within themselves. Yes, they get more complicated, but if you can do basic chemical equations, you will know what to do with the crazier ones that take more effort and eraser dust.
___ N2 + ____H2 ------> ___NH3
My attention immediately goes to the 3 Hydrogen atoms in NH3 since it's the most abundant atom. There is no number that we can put in front of the H2 to make it equal to the 3 Hydrogens on the right side.
The first thing I think to do is multiply the subscripts to produce a number they both will divide into. 3x2=6 and we can get 6 Hydrogen atoms on each side by adding a 3 on the left side and a 2 on the product side:
___ N2 + 3H2 ------> 2NH3
Now count everything. We have 2 Nitrogen atoms and 6 Hydrogen atoms on the left side. And we have 2 Nitrogen atoms and 6 Hydrogen atoms on the right side.
What? We're done? Yep. Here's the balanced equation.
N2 + 3H2 ------> 2NH3
___S8 + ___O2 -------> ____SO3
You can probably predict where my eyes go. My focus goes to the S8 since it has the most atoms. We need to get 8 Sulfur on the right side, too. So just put an 8 over there.
___S8 + ___O2 -------> 8SO3
Now you have 8 Sulfur atoms and 2 Oxygen atoms on the left. We have 8 Sulfur atoms and 24 Oxygen atoms on the right. The Sulfur is balanced on both sides, but the Oxygen is not. We now need 24 Oxygen atoms on the left. We do that by placing a 12 in front of the O2.
S8 + 12 O2 -------> 8SO3
Bam! Balanced equation.
___N2 + ___O2 -------> ____ N2O
Alright. We can already see that there is the same amount of Nitrogen on each side (2 atoms). So we have to deal with the Oxygen because there are 2 Oxygen atoms on the left reactant side but only one on the right.
So let's add a 2 to the right to balance the Oxygen.
___N2 + ___O2 -------> 2N2O
In doing this, we've changed the amount of Nitrogen on the right of the arrow so that its no longer balanced. Now there are 2 Nitrogen atoms on the left and 4 Nitrogen atoms on the right. To fix this we simply must add a 2 to the reactant Nitrogen on the left like so:
2N2 + O2 -------> 2N2O
And we are done!
___HgO ------> ____Hg + O2
This one is really easy. Let's balance the Oxygen. There are 2 Oxygen on the right. Just add 2 to the HgO to balance the Oxygen to have 2 atoms on each side of the arrow.
2HgO ------> ____Hg + O2
In doing this, we've increased the Mercury (Hg) to 2 atoms. All we must do is add 2 to the Hg on the right to balance this problem.
2HgO ------> 2Hg + O2
And Voila!
____CO2 + ____H2O -----> ___C6H12O6 +____ O2
Uh oh. This one looks a little intimidating, right? Don't worry. Just keep count and break it down.
I'd tackle the 12 Hydrogen in the C6H12O6 molecule first. How do we get 12 Hydrogen on the left side? All we do is put a 6 in front of the H2O.
____CO2 + 6H2O -----> ___C6H12O6 +____ O2
Now on the left side we have: 1 Carbon atom, 8 Oxygen atoms and 12 Hydrogen atoms
And on the right side we have: 6 Carbon atoms, 8 Oxygen atoms and 12 Hydrogen atoms.
What I would do is then add a 6 to the CO2.
6CO2 + 6H2O -----> ___C6H12O6 +____ O2
Now on the left we have: 6 Carbon atoms, 18 Oxygen atoms and 12 Hydrogen atoms.
And on the right we still have our 6 Carbon atoms, 8 Oxygen atoms and 12 Hydrogen atoms.
So how do we balance out the Oxygen? Well, we need to get 18 Oxygen on the right. Just add 6 to the O2 on the right.
6CO2 + 6H2O -----> C6H12O6 + 6O2
Now on the left: 6 Carbon, 18 Oxygen, 12 Hydrogen
And on the right: 6 Carbon, 18 Oxygen, 12 Hydrogen
I call that balanced.
___Zn + __HCl -------> ____ZnCl2 + ___ H2
After that one, this one should be super easy. Add a 2 to the HCl to balance the Chlorine.
Zn + 2HCl -------> ZnCl2 + H2
There are: 1 Zinc atom, 2 Hydrogen atoms and 2 Chloride atoms on both sides---and we have a balanced equation.
Wednesday, November 14, 2012
Tuesday, November 13, 2012
How to Balance Chemical Equations Step By Step
Balancing chemical equations can be one of the most confusing things for new chemistry students. Everyone doesn't have the luxury of a good teacher who will take the time to explain it for those who just don't get it. I'm not saying I'm the best, but maybe the following will help it click for someone who is just confused.
When chemical reactions occur, most of the time the atoms in the resulting product molecules are not in the same amount as the individual reactants.
Here is a skeleton view of a basic chemical reaction:
X + Y ----------> XY
Although it looks like one X and one Y should also be the same on the other side, the product molecule may contain varying amounts of each atom.
For example:
H2 + O2 -------------> H2O
On the reactant side are two Hydrogen atoms and two Oxygen atoms. When reacted they yield H2O molecules.
Although we start out with 2 Hydrogen atoms and 2 Oxygen atoms, the resulting H2O molecule only contains 1 Oxygen atom. We must balance the equation so that there is the same number of EACH atom on BOTH sides.
The balanced version of this equation would look like this:
2H2 +O2 --------------> 2H2O
You can count and see that we have 4 H and 2 O on the reactant side and we also have 4 H and 2 O on the product side.
(Note: The coefficient 2 means that there are two molecules of Hydrogen and 2 molecules of H2O. Therefore, you would multiply the atoms of each molecule by the coefficient to calculate the number of atoms present.)
I'm going to post a couple equations and just share my mental process in solving them. As you learn to balance chemical reactions, keep the following in mind:
1.When you balance equations, the only numbers you ever change are the coefficients (the number in front of the molecule). Never change the subscripts of the molecules. This would make them different substances!
Note that
H4 = 4 atoms of Hydrogen
4H = 4 atoms of Hydrogen
2H2 = 4 atoms of Hydrogen
If there is no coefficient or subscript, the atom is just counted once.
2. Work these out in pencil. You will likely have to erase more than once before you correctly balance these reactions.
3. It may be helpful to write out how many atoms of each element are on each side as you go along.
4. Remember that the end goal is to have the same amount of each element on both sides of the arrow.
1. ____ H2 + ____ O2 --------> ______H2O
When I begin my process of balancing an equation, my attention immediately goes to the atom in largest quantity. You know that you have to have to balance the equation to have at least that many atoms on the other side.
Since the Hydrogen atoms are already balanced on both sides (there are 2 Hydrogen atoms on each side), let's look at the O2 on the reactant side.
There are 2 Oxygen atoms on the left side, right? This means you must make the Oxygen atoms on the product side equal 2, also. Do this by placing a 2 in front of the H2O product.
_____ H2 + _____O2 -----------> 2 H2O
Now we have 2 Oxygen atoms on the left and 2 Oxygen atoms on the right side. But guess what happened when we did this. When we added the 2 in front of the H2O we increased the number of Oxygen atoms, but we also increased the number of Hydrogen atoms.
There are now 4 Hydrogen atoms on the right side.
What to do? Well, we have to make it so that there are 4 Hydrogen atoms on the left side, too. So, simply add a 2 in front of the Hydrogen molecule on the left to get this:
2 H2 + ___ O2 ------> 2 H2O
Now if you count everything, you see that there are 4 Hydrogen atoms on both sides and 2 Oxygen atoms on both sides. Therefore this chemical equation is balanced. We don't even have to change or add a coefficient to the O2 on the left side. Our work is done.
2 H2 + O2 -------> 2 H2O
Here are a few more equations for you to try. Find the answers and my personal process in solving them in my next post Balancing Equations For Chemistry.
___ N2 + ____H2 ------> ___NH3
___S8 + ___O2 -------> ____SO3
___N2 + ___O2 -------> ____ N2O
___HgO ------> ____Hg + O2
____CO2 + ____H2O -----> ___C6H12O6 +____ O2
___Zn + __HCl -------> ____ZnCl2 + ___ H2
When chemical reactions occur, most of the time the atoms in the resulting product molecules are not in the same amount as the individual reactants.
Here is a skeleton view of a basic chemical reaction:
X + Y ----------> XY
Although it looks like one X and one Y should also be the same on the other side, the product molecule may contain varying amounts of each atom.
For example:
H2 + O2 -------------> H2O
On the reactant side are two Hydrogen atoms and two Oxygen atoms. When reacted they yield H2O molecules.
Although we start out with 2 Hydrogen atoms and 2 Oxygen atoms, the resulting H2O molecule only contains 1 Oxygen atom. We must balance the equation so that there is the same number of EACH atom on BOTH sides.
The balanced version of this equation would look like this:
2H2 +O2 --------------> 2H2O
You can count and see that we have 4 H and 2 O on the reactant side and we also have 4 H and 2 O on the product side.
(Note: The coefficient 2 means that there are two molecules of Hydrogen and 2 molecules of H2O. Therefore, you would multiply the atoms of each molecule by the coefficient to calculate the number of atoms present.)
I'm going to post a couple equations and just share my mental process in solving them. As you learn to balance chemical reactions, keep the following in mind:
1.When you balance equations, the only numbers you ever change are the coefficients (the number in front of the molecule). Never change the subscripts of the molecules. This would make them different substances!
Note that
H4 = 4 atoms of Hydrogen
4H = 4 atoms of Hydrogen
2H2 = 4 atoms of Hydrogen
If there is no coefficient or subscript, the atom is just counted once.
2. Work these out in pencil. You will likely have to erase more than once before you correctly balance these reactions.
3. It may be helpful to write out how many atoms of each element are on each side as you go along.
4. Remember that the end goal is to have the same amount of each element on both sides of the arrow.
1. ____ H2 + ____ O2 --------> ______H2O
When I begin my process of balancing an equation, my attention immediately goes to the atom in largest quantity. You know that you have to have to balance the equation to have at least that many atoms on the other side.
Since the Hydrogen atoms are already balanced on both sides (there are 2 Hydrogen atoms on each side), let's look at the O2 on the reactant side.
There are 2 Oxygen atoms on the left side, right? This means you must make the Oxygen atoms on the product side equal 2, also. Do this by placing a 2 in front of the H2O product.
_____ H2 + _____O2 -----------> 2 H2O
Now we have 2 Oxygen atoms on the left and 2 Oxygen atoms on the right side. But guess what happened when we did this. When we added the 2 in front of the H2O we increased the number of Oxygen atoms, but we also increased the number of Hydrogen atoms.
There are now 4 Hydrogen atoms on the right side.
What to do? Well, we have to make it so that there are 4 Hydrogen atoms on the left side, too. So, simply add a 2 in front of the Hydrogen molecule on the left to get this:
2 H2 + ___ O2 ------> 2 H2O
Now if you count everything, you see that there are 4 Hydrogen atoms on both sides and 2 Oxygen atoms on both sides. Therefore this chemical equation is balanced. We don't even have to change or add a coefficient to the O2 on the left side. Our work is done.
2 H2 + O2 -------> 2 H2O
Here are a few more equations for you to try. Find the answers and my personal process in solving them in my next post Balancing Equations For Chemistry.
___ N2 + ____H2 ------> ___NH3
___S8 + ___O2 -------> ____SO3
___N2 + ___O2 -------> ____ N2O
___HgO ------> ____Hg + O2
____CO2 + ____H2O -----> ___C6H12O6 +____ O2
___Zn + __HCl -------> ____ZnCl2 + ___ H2
Monday, November 12, 2012
Molar Mass to Molecular Mass
Finding the molecular mass of a molecule is as simple as adding the molar mass of all the involved elements. Molecular mass is actually a misnomer and some tend to use it interchangeably with molecular weight. I'm not a chemist, so they both mean the same thing to me and I've seen both terms used.
First of all, a molecule is the combination of more than one atom (of the same or different elements) to form a substance. H (hydrogen) is an element. H2O, O2 and HCl are molecules.
The molecular mass is simply the weight of one molecule.
To find the molecular mass, simply add up the weights of every individually present element.
For example, a molecule of H2O contains 1 atom of Hydrogen and 2 atoms of Oxygen.
It's really not as intimidating as it may seem. Just add up every element in the molecule. Your biggest problem may be overlooking the actual number of atoms, so be careful.
First of all, a molecule is the combination of more than one atom (of the same or different elements) to form a substance. H (hydrogen) is an element. H2O, O2 and HCl are molecules.
The molecular mass is simply the weight of one molecule.
To find the molecular mass, simply add up the weights of every individually present element.
For example, a molecule of H2O contains 1 atom of Hydrogen and 2 atoms of Oxygen.
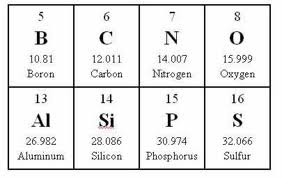
If you look at your periodic table you will see the atomic mass for each element under the chemical symbol.
(Note: Your periodic chart likely has atomic masses that extend several places past the decimal. The numbers here are rounded up. Use the weights that you see in your book, the concept is the same.)
Alright. The molecular mass of one molecule of H2O is 18 g/mol. There are 2 Hydrogen atoms (1 gram x2=2) and 1 Oxygen atom (16 grams). 2+16=18.
Let's do the same with O2. There are 2 atoms of Oxygen (16 g x 2) in this molecule, so its molecular weight is 32 g/mol.
One more?
HCl has one atom of Hydrogen (1g) and one atom of Chlorine.(35 g), so the molecular weight of HCl is 36g/mol. (1 g +35 g)
Determining Valence Electrons
Valence electrons are those in the outermost electron shell of an atom. These special electrons determine the atoms reactivity because they are free to form bonds with the valence electrons of other atoms. An atom's life goal is to achieve 8 electrons in its outer shell through bonding with other atoms. When the atom has 8 electrons in it's outer shell it is stable.
Valence is important when determining how to draw Lewis Structures.
Let's try to start from the beginning. I haven't been in a chemistry class for a while, so this is going to be basic, but hopefully helpful.
Okay, so you may know that an atom is comprised of protons, neutrons and electrons. The protons and neutrons form the atom's nucleus, while the electrons float around it.
If you look at an element on the periodic table, you will see that each one has an atomic number.
Carbon's atomic number is 6. Nitrogen's atomic number is 7. The atomic number also represents the number of protons and electrons of the element. For example, Carbon is made up of 6 protons and 6 electrons. Likewise, Nitrogen has 7 protons and 7 electrons.
We are trying to determine valence electrons so let's do it.
Things get kind of tricky, the higher you go up the periodic table so we are going to stick with the lower numbers just so you understand the concept of how the valence is determined.
Look at my little ghetto drawing above. Microsoft Paint, what!
Anyway, as you can see the protons/neutrons form the nucleus in the middle and there are electrons hanging about on the surrounding rings.
The first electron ring can hold 2 electrons. The second electron ring can hold 8. And the third ring will hold 8 (sometimes more, but we're going to keep it simple).
Carbon.
Carbon has 6 electrons (look at the atomic number). This means that we have 6 electrons to place in the surrounding electron shells.
Therefore, there will be 2 electrons in the first shell and 4 electrons in the second shell. Since there are 4 electrons in the outer shell Carbon's valence is 4.
How about another?
Oxygen has 8 electrons. This means that 2 electrons would go in the first electron shell and 6 electrons would go in the outer shell. This is why Oxygen has a valence of 6.
Let's do one with a higher number. Phosphorus.
Phosphorus has 15 electrons. So it has 2 electrons in its first shell, 8 electrons in the second shell and 7 electrons in its outer valence shell.
Get it? Read through and look at the diagram above and you'll have it!
Valence is important when determining how to draw Lewis Structures.
Let's try to start from the beginning. I haven't been in a chemistry class for a while, so this is going to be basic, but hopefully helpful.
Okay, so you may know that an atom is comprised of protons, neutrons and electrons. The protons and neutrons form the atom's nucleus, while the electrons float around it.
If you look at an element on the periodic table, you will see that each one has an atomic number.
Carbon's atomic number is 6. Nitrogen's atomic number is 7. The atomic number also represents the number of protons and electrons of the element. For example, Carbon is made up of 6 protons and 6 electrons. Likewise, Nitrogen has 7 protons and 7 electrons.
We are trying to determine valence electrons so let's do it.
Things get kind of tricky, the higher you go up the periodic table so we are going to stick with the lower numbers just so you understand the concept of how the valence is determined.
Look at my little ghetto drawing above. Microsoft Paint, what!
Anyway, as you can see the protons/neutrons form the nucleus in the middle and there are electrons hanging about on the surrounding rings.
The first electron ring can hold 2 electrons. The second electron ring can hold 8. And the third ring will hold 8 (sometimes more, but we're going to keep it simple).
Carbon.
Carbon has 6 electrons (look at the atomic number). This means that we have 6 electrons to place in the surrounding electron shells.
Therefore, there will be 2 electrons in the first shell and 4 electrons in the second shell. Since there are 4 electrons in the outer shell Carbon's valence is 4.
How about another?
Oxygen has 8 electrons. This means that 2 electrons would go in the first electron shell and 6 electrons would go in the outer shell. This is why Oxygen has a valence of 6.
Let's do one with a higher number. Phosphorus.
Phosphorus has 15 electrons. So it has 2 electrons in its first shell, 8 electrons in the second shell and 7 electrons in its outer valence shell.
Get it? Read through and look at the diagram above and you'll have it!
How to Do Lewis Dot Structure (Simple)
The Lewis Dot Electron Diagram Explained
If you can figure out the number of valence electrons surrounding your atom, the construction of the Lewis dot forms is easy. Here is a makeshift outline of the periodic table showing the number of valence electrons for each group of elements. Don't laugh at my picture.
 The elements in group 1A have one electron in their outer valence shells and the elements in group 7A have seven electrons in their valence shell.
The elements in group 1A have one electron in their outer valence shells and the elements in group 7A have seven electrons in their valence shell.
(Elements in group 8A have eight electrons in their outer shells, but they are stable and non reactive because their outer shells are complete and happy with 8 electrons. More HERE.)
So, let's choose the element Lithium in group 1A. How many valence electrons does it have?
One. Right?
To draw the Lewis Structure for Lithium, we must draw a dot representing its one valence electron around its chemical symbol. It would look like this:
That was easy.
Now how about Nitrogen? Look at your periodic table. Nitrogen is in group 5A, so how many valence electrons does it have? Yep, Five.
We need to represent five electrons around the chemical symbol for Nitrogen, so its Lewis structure would look like this:
The easiest way to place the electrons is to draw one dot at a time starting at the top going clockwise around the symbol. Let's say that there are four "poles" (top, right, bottom, left). For now there should never be more than two dots (electrons) at each pole. And if there are two dots at a pole there should at least be one dot at the other poles.
In other words, don't do this:
Make sure every pole has at least one electron before you go doubling up at the other ends.
If you can figure out the number of valence electrons surrounding your atom, the construction of the Lewis dot forms is easy. Here is a makeshift outline of the periodic table showing the number of valence electrons for each group of elements. Don't laugh at my picture.
 The elements in group 1A have one electron in their outer valence shells and the elements in group 7A have seven electrons in their valence shell.
The elements in group 1A have one electron in their outer valence shells and the elements in group 7A have seven electrons in their valence shell.(Elements in group 8A have eight electrons in their outer shells, but they are stable and non reactive because their outer shells are complete and happy with 8 electrons. More HERE.)
So, let's choose the element Lithium in group 1A. How many valence electrons does it have?
One. Right?
To draw the Lewis Structure for Lithium, we must draw a dot representing its one valence electron around its chemical symbol. It would look like this:
Now how about Nitrogen? Look at your periodic table. Nitrogen is in group 5A, so how many valence electrons does it have? Yep, Five.
We need to represent five electrons around the chemical symbol for Nitrogen, so its Lewis structure would look like this:
The easiest way to place the electrons is to draw one dot at a time starting at the top going clockwise around the symbol. Let's say that there are four "poles" (top, right, bottom, left). For now there should never be more than two dots (electrons) at each pole. And if there are two dots at a pole there should at least be one dot at the other poles.
In other words, don't do this:
Make sure every pole has at least one electron before you go doubling up at the other ends.
Subscribe to:
Comments (Atom)